




-
Project
Name: SonoSite SII
Development: 2014 - 2015
Role: Lead Industrial Designer
Design Leadership: Craig Chamberlain, David Wykes
Team: Ben Dekock, Evan MacCormick, Tyler Dawson
Awards: Good Design Award 2017
IDEA Silver 2016
Good Design - Gold 2016 (Chicago Athenaeum)Patents: USD796679S1, USD796678S1
-
Overview
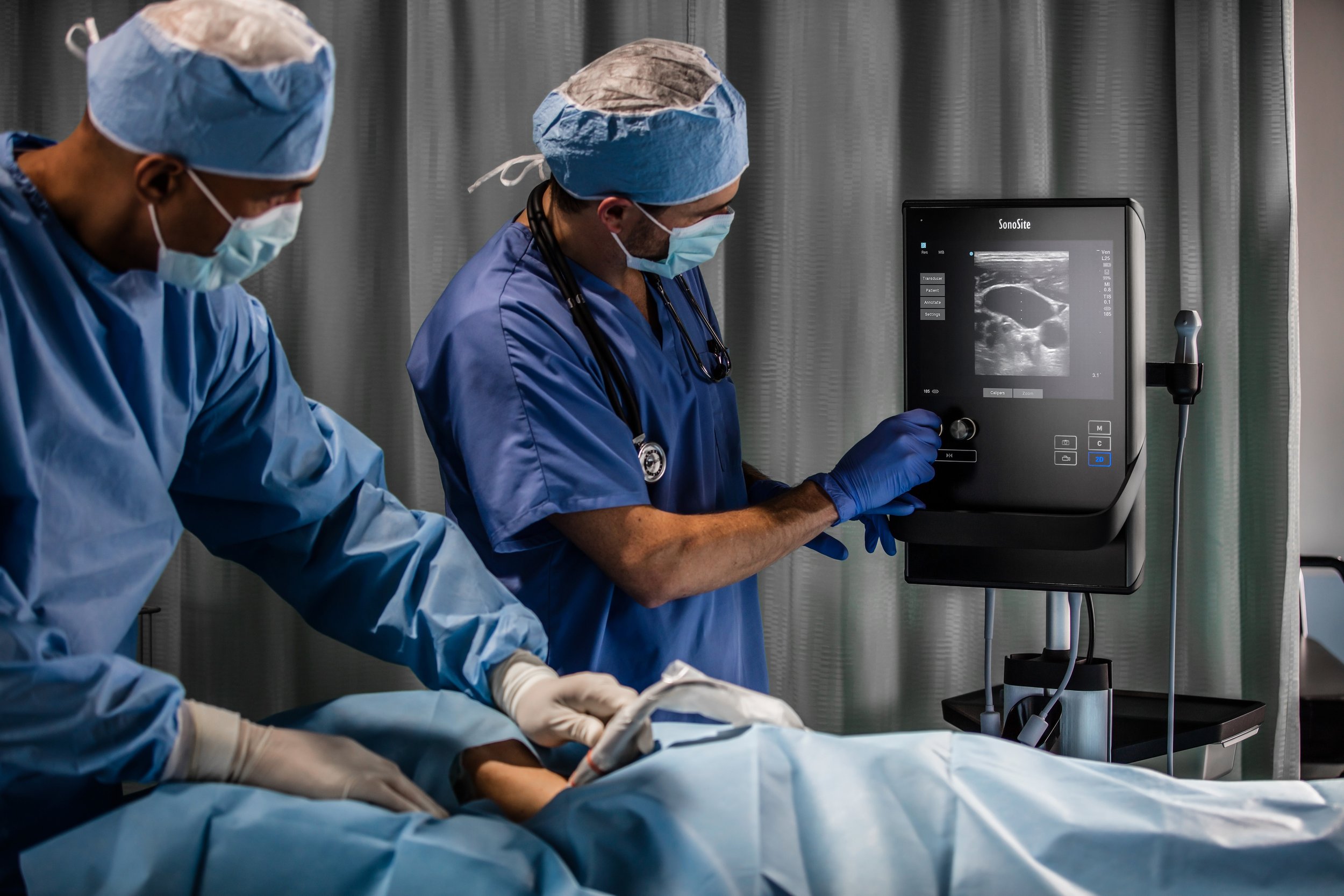
In 2014, SonoSite kicked off the industrial design effort for the SII, a portable ultrasound machine designed to serve anesthesiologists and other procedural disciplines. Procedural ultrasound is all about performing a specified task, such as a regional nerve block prior to surgery. The hardware and software are optimized for a specific workflow and prioritize certain functions over others.
What is point-of-care ultrasound?
Ultrasound allows for a non-invasive window into the human body. Point-of-care (POC) ultrasound is where the device is brought to the patient’s bedside (the point of care), rather than the patient going to the device in its permanent location in the hospital or clinic. This reduces the amount of time it takes for a physician to get the answers needed to determine a patient’s course of treatment, and in emergency scenarios could be the difference between life and death. Point-of-care ultrasound serves three major medical subspecialties: emergency medicine, critical care, and anesthesiology. -
Design
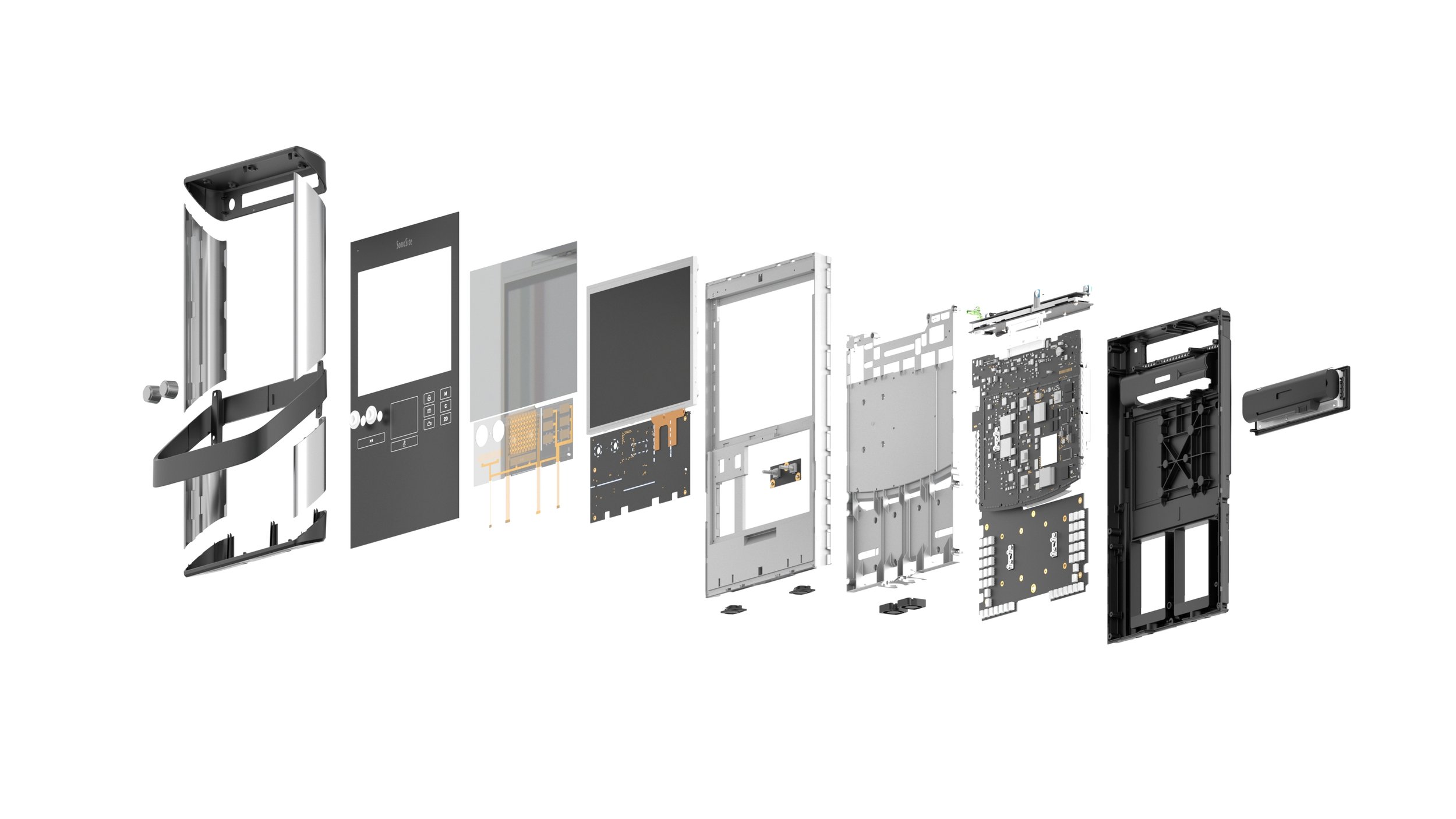
The SII has a unique vertical tablet inspired form factor that is both physically and visually thin. This allows for doctors to fit the device into the limited space around the patient’s bedside. The device has an integrated touch screen and persistent control panel. Bringing the most important buttons and functions (depth, gain, and modes) forward and placing them in a prominent location for the user to utilize with minimal barriers. Drawing on the previous Turbo-S ultrasound machine, the depth and gain remain as tactile rotary knobs that allow for precision control, but now protrude through the one piece chem-strengthened cover glass. The SII has an integrated dual transducer connector (DTC), that allows users to connect multiple transducers and switch between them seamlessly depending on the depth and type of imaging necessary.
The overall structure and chassis of the machine is made from magnesium, with extruded aluminum sleeves added to the exterior sides of the device to increase strength and robustness. The SII has a single piece cast magnesium handle that provides not only a place to grab and manipulate the angle and rotation of the device, but also protects it from frontal impact while maneuvering throughout the hospital. Special care was given to infection control by minimizing the gaps and number of part lines present on the device, and by the use of chemically resistant materials and finishes. The cart system offers a simple yet unique way of holding the transducers up high on the device to offer protection from being run over by the cart wheels, and to simplify the sterile sheathing process if necessary. The tray on the cart was moved to the rear to allow for the entire system to get closer to the bedside, and bespoke gel storage was added to the front.
-
Color, Material, Finish
Main housings: Magnesium, painted chemical resistant black
Side panels: Extruded aluminum, Anodized 70% gray, vertical brush
Top caps: Injection molded Tritan, Fire resistant black, MT11020
Handle: Solid magnesium, painted chemical resistant black
Stand column: Extruded aluminum, anodized clear, vertical brush
Stand components: Die cast aluminum, powder coated black.